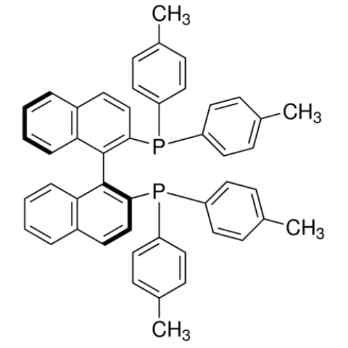
(S)-T-BINAP
Asymmetric Synthesis, BINAPs, Chemical Synthesis, Chiral Catalysts, Ligands, and Reagents, Privileged Ligands and Complexes, BINAPs
(S)-T-BINAP is a versatile chiral organophosphorus ligand and has been used in several catalytic applications such as:
1. Palladium catalyzed carbon-oxygen bond formation (Ref: J. Am. Chem. Soc., 1997, 119, 11108)
2. Palladium-catalyzed α-arylation reaction of ketones (Ref: J. Am. Chem. Soc., 2000, 122, 6797)
3. Cu-catalyzed asymmetric conjugate reduction (Ref: J. Am. Chem. Soc., 1998, 120, 837)
4. Cu-catalyzed asymmetric dienolate addition to aldehydes (Ref: J. Am. Chem. Soc., 2003, 125, 11253)
5. Enantioselective conjugate reduction of lactones and lactams (Ref: Org. Lett., 2003, 5(20), 3691)
6. Enantioselective cycloaddition of allenylsilanes with α-Imino esters (Ref: J. Am. Chem. Soc., 2003, 125, 5644)
7. Catalytic Aldol reaction to ketones (Ref: Org. Lett., 2005, 7(22), 4955)
8. Rhodium catalyzed [2+2+2] cycloaddition reaction of alkenes and alkynes (Org. Lett., 2011, 13, 4692)
9. Iridium-catalyzed enantioselective C-H bond activation of 2-(alkylamino)-pyridine with alkenes (Ref: J. Am. Chem. Soc., 2014, 136, 8911)
10. Iridium-catalyzed regio-, diastereo-, and enantioselective tert-(hydroxyl)-prenylation of alcohols (Ref: J. Am. Chem. Soc., 2014, 136, 8911)
11. Rhodium-catalyzed cross cyclotrimerization (Angew. Chem., Int. Ed., 2014, 53, 2956)
Note: Please check notes for S-BINAP for additional applications
Price and Availability
| Quantity | Availability | *Price (INR) |
|---|---|---|
| 1 gm | In Stock | On Request |
| 10 gm | In Stock | On Request |
| 50 gm | In Stock | On Request |
| 1 kg | 4-5 Weeks | On Request |
| 25 kg | 8-10 Weeks (Negotiable) | On Request |
For bulk inquiries and more details please email us on
QUICK CONTACT
If you have any questions or would like to book a session please contact us.